Hyundai Bioland Logo
Advanced Regenerative Medicine
 Advanced Regenerative Medicine
Advanced Regenerative Medicine
・ Key Materials
Dentistry
Collagen /
Bone graft material

Technology for regenerating
periodontal tissue (FDA, CE)
Collagen membrane ¹⁾
Induce the regeneration of collagen-based barrier membrane and alveolar bone
Bone graft material
Promote the regeneration of bone and collagen-based bone graft material
and alveolar bone ²⁾


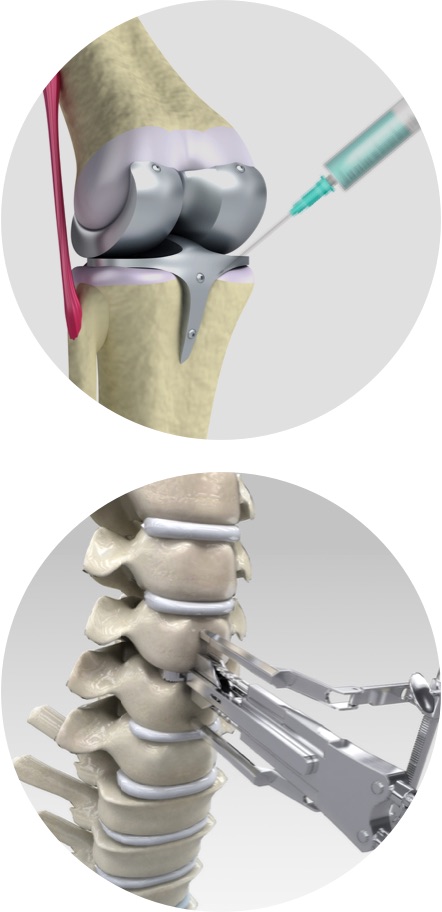
Orthopedics
Collagen / BioCeramic /
Polymer

Technological development
of replacements forbone
and cartilage defects
Technology for forming collagen injection for tissue regeneration
Collagen-based replacement of ligament and tendon defects
Prosthetics/vertebroplasty bone cement
PMMA, MMA and gentamicin combined technology
Implant fixed on bone
Synthetic bone graft materials
for the formation of new bones
Maintain stable volume using β-TCP+HAp
Secure excellent bone absorption and regeneration technology

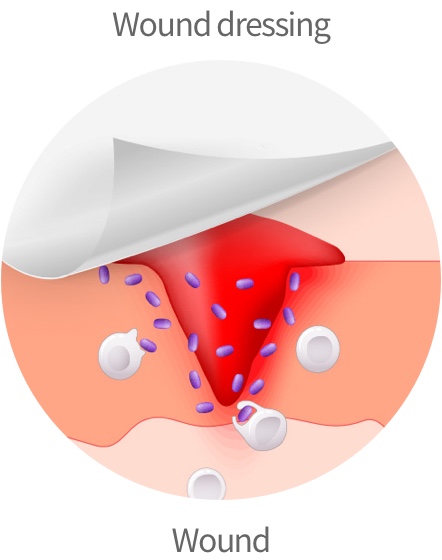
Dermatology

Collagen /
Hyaluronic acid / Silicone

Securing technologies for medical
materials for injuries, scars,
and beauty purposes
Wound dressing³⁾ for healing burns and wounds
Technology for manufacturing collagen and hyaluronic acid-based wound dressing
Artificial dermis and promotion of dermal regeneration for burn treatment
Silicone-material technology excellent in scar treatment
Protection and recovery of scars through silicone hardening technology

¹⁾ Collagen membrane: Protective layer to cover the affected area after implant and soft tissue surgery
²⁾ Alveolar bone graft material: Heterogeneous bone graft material for dentistry
³⁾ Wound dressing: Medical material that helps in the regeneration of damaged skin due to burns or ulcer
・ Advanced Surgical Hemostats


Regeneration


Cartilage Regeneration Treatment
Establishment of clinical research competency and pipeline through open innovation.
Indication expansion of Cartistem1)
for treatment of cartilage defects on ankle joint.
1) Cartistem: Stem cell therapeutic product for knee cartilage regeneration in patients with defected cartilage.
It is developed and manufactured by MEDIPOST Co., Ltd.
Phase 3 clinical trial is ongoing in Korea.
Immune Modulation

Atopic Dermatitis Treatment
Securing manufacturing license and pipeline through open innovation
Treatment for moderate-to-severe atopic dermatitis via immune and inflammation modulation.
Core Platform
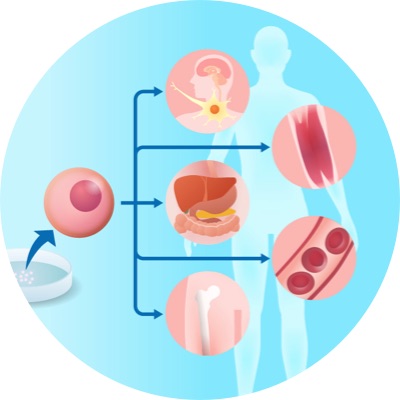

HDB Future Bio Core Technology
Development of HDB’s Cell therapy platform with multifunction and high efficiency.
Establishment of technologies for activating and controlling the function of cellular active ingredients
Maximization of therapeutic efficacy by strengthening anti-inflammatory and tissue regeneration functions
Expansion of pipeline based on definitive mechanism analysis
(cardiovascular system, central nervous system, etc.)